TL;DR - Executive Summary
- In the UK, cannabis-based products for medicinal use (CBPMs) are classified a Class B drug under Schedule 2 and prescribed only by GMC-registered specialists. Recreational use of cannabis remains illegal.
- Unlicensed CBPM prescriptions in the private sector are quickly rising – especially in the past 3 years. However, CBPM prescriptions by the NHS are negligible – only under 5 a month.
- CBPM prescriptions in the private sector have risen 130% in 2024 compared to 2023, according to a report by the Care Quality Commission (CQC)
- The percentage growth of prescriptions for CBPMs exceed those of mainstream drugs such as Atorvastatin, Amlodipine, Omeprazole and Ramipril.
- However, the CQC expresses its concerns around poor communication & coordination between providers, GPs and the patients themselves, the questionable safety & legality of current prescriptions, the lack of documented evidence justifying a CBPM will fulfill an unmet clinical need, and the adherence to ethical advertising practices.
- Growth for UK’s medical cannabis industry is promising – but this should not come at the cost of compliance to ensure safety & quality of life for all patients.
- Guidance on how to prove you have a CBPM prescription is provided as well as a link to access a NHS’s CBPM e-learning package (only for UK healthcare professionals).
Table of Contents
A 2025 Overview of Medicinal Cannabis in the UK
In the UK, cannabis is only permitted for medicinal means – while still remaining illegal for recreational use.
In 2018, several reviews were conducted by the British government into cannabis and cannabis-related products resulting in evidence that supported its medicinal benefit for certain conditions.
As a result, the government announced its intentions to allow providers to prescribe cannabis-based products for medicinal use (CBPMs) with the first move being to reclassify it from Schedule 1 to Schedule 2 of the Misuse of Drugs Regulations 2001.
It is also important to note – as they may be easily confused – that CBPMs are an entirely distinct category from ‘cannabis-based medicines’ (CBMs).
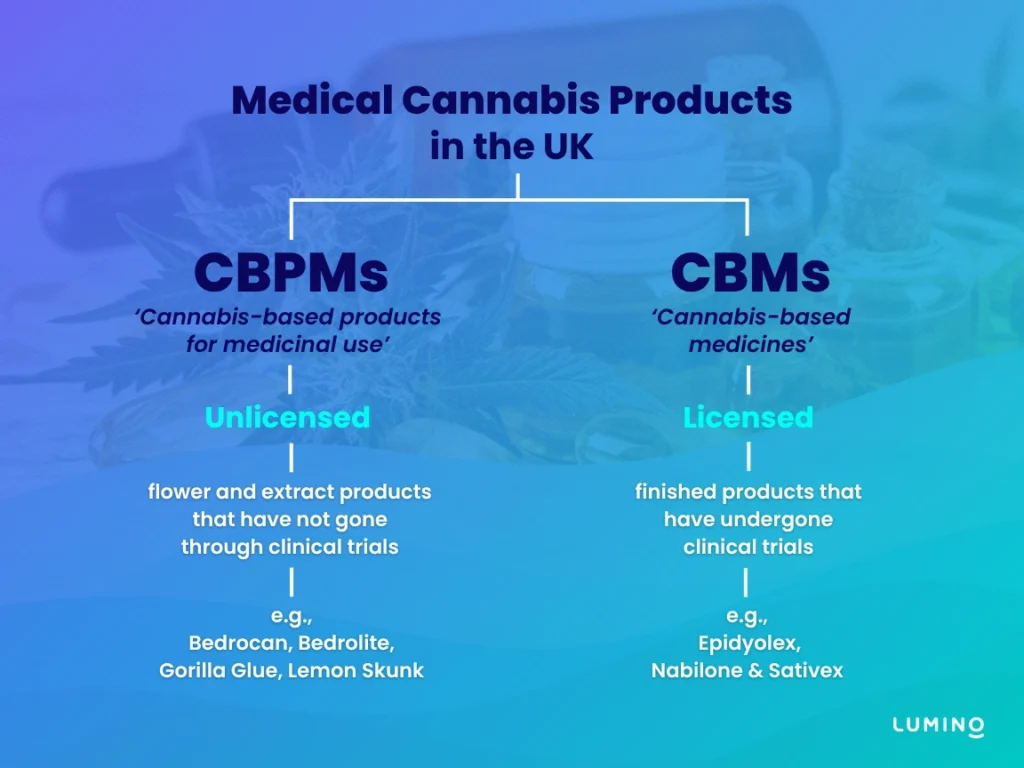
How are CBMs different from CBPMs?
Distinct from CBPMs, ‘Cannabis-based medicines’ (CBMs) are products that:
- are categorized and scheduled separately under the Misuse of Drugs Regulations
- have received marketing authorization from the medicines regulator (either the MHRA or the EMA)
- are licensed pharmaceutical products
- can be prescribed by any General Practitioner (GP) – however, the lack of NHS authorisations and education hinders any growth in CBM prescriptions.
Currently, there exists only 3 CBMs in the UK, namely: Epidyolex, Nabilone, and Sativex. However, despite their legality & availability, the NHS strongly advises doctors not to freely prescribe them.
'Licensed' vs 'Unlicensed CBPMs'
‘Licensed CBPMs’ pertains to products that have received marketing authorisation from the medicines regulator (either the MHRA or the EMA), yet still meet the criteria of a CBPM under Regulation 2(1) of the MDR. As of 2025, the UK has no registered licensed CBPM. It is generally understood that a licensed CBPM – passing rigorous clinical trial and receiving marketing authorisation – will most likely be classified as a CBM.
‘Unlicensed CBPMs’, on the other hand, refers to products that meet the definition of a CBPM, but have not been evaluated for safety, quality or efficacy by regulatory bodies, such as the Medicines and Healthcare products Regulatory Agency (MHRA) or the European Medicines Agency (EMA).
The MHA offers guidance on manufacturing, importing, distributing or supplying unlicensed CBPMs.
Who can prescribe CBPMs?
According to the General Medical Council, CBPM prescriptions can only be made:
- By doctors on the specialist register of the General Medical Council
- If it lies within the specialist doctor’s own area of practice and training/limits of competence
- If the decision has been agreed upon by a multidisciplinary team
- When there is no other suitably licensed medicine available that will meet the patient’s need.
If you are a healthcare professional wanting to learn more, the National Health Service (NHS) England have developed an e-learning package in 2018 in collaboration with the Health Education England and the University of Birmingham.
Cannabis-based products remaining under Schedule 1
Any cannabis-based products not meeting the Misuse of Drugs Regulations 2001’s criteria (other than cannabis-based medicines that have received marketing authorisation and have been separately scheduled) remain classified under Schedule 1, meaning it is unlawful to possess, supply, offer to supply, produce, import or export this drug except under a Home Office licence.
Any Growth in CBPM Prescriptions Since 2018's Legalisation?
Patients being turned away by the NHS
In the public sector, despite the 2018 law change, it has proven difficult to acquire medical cannabis from the NHS – especially for CBPMs.
When looking at CBMs (i.e., Epidyolex, Nabilone & Sativex), a statement by the UK’s Department of Health & Social Care reveals a steady increase in the number of NHS prescriptions for licensed cannabis-based medicines since 2018, with a 16% dip in 2024 (as seen in the diagram below).
However, these numbers are relatively low considering how in 2023 – the year with a noticeable spike – there were only 1,104 patients prescribed CBMs, an increase of merely 127 patients compared to 2021.
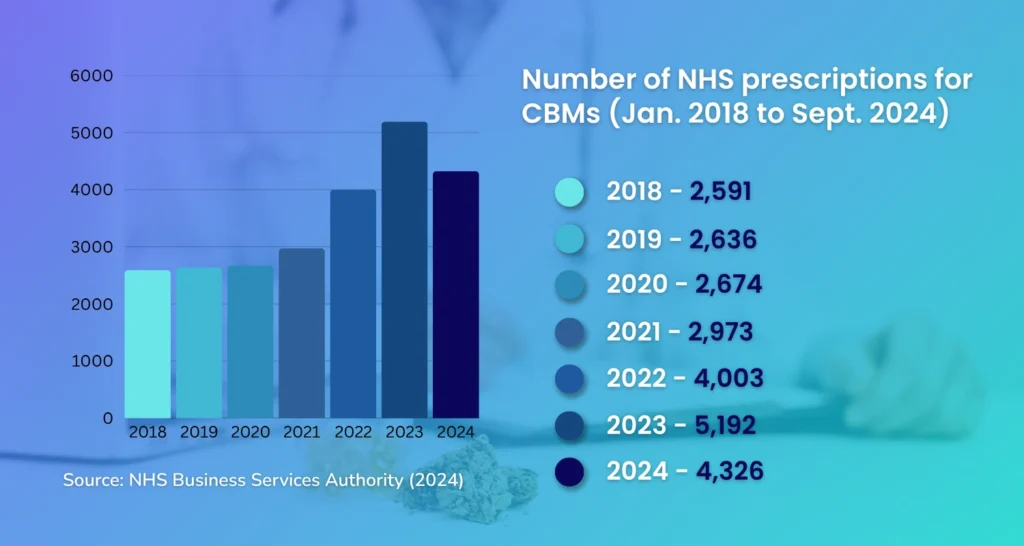
When looking specifically at CBPMs, on the other hand, the number of NHS prescriptions for cannabis-based products for medicinal use were virtually negligible – often fewer than 5 per month – as revealed by former Health Minister, Will Quince in 2023.
These prescriptions went out to just a handful of children with extremely rare, treatment-resistant epilepsies not controllable by standard therapies, such as a four-year old girl with Aicardi syndrome, a rare genetic neurological disorder.
The reasons?
- Most doctors lacked knowledge of medical cannabis, and/or still saw the stigma associated with it.
- Some specialists who are aware of it argue that there is not enough evidence regarding the drug’s safety and effectiveness (i.e., lack of randomized controlled trial (RCT) data) to justify its prescription.
- The specialists who do want to prescribe unlicensed cannabis-based products almost always get turned down for funding by the NHS.
- NHS trusts often refer patients to private clinics.
Naturally, many patients, believing CBPMs could be freely prescribed by specialist doctors, felt misled. And as a result, they had to either forego, or pay privately.
The Resulting Boom in Private Sector CBPM prescriptions
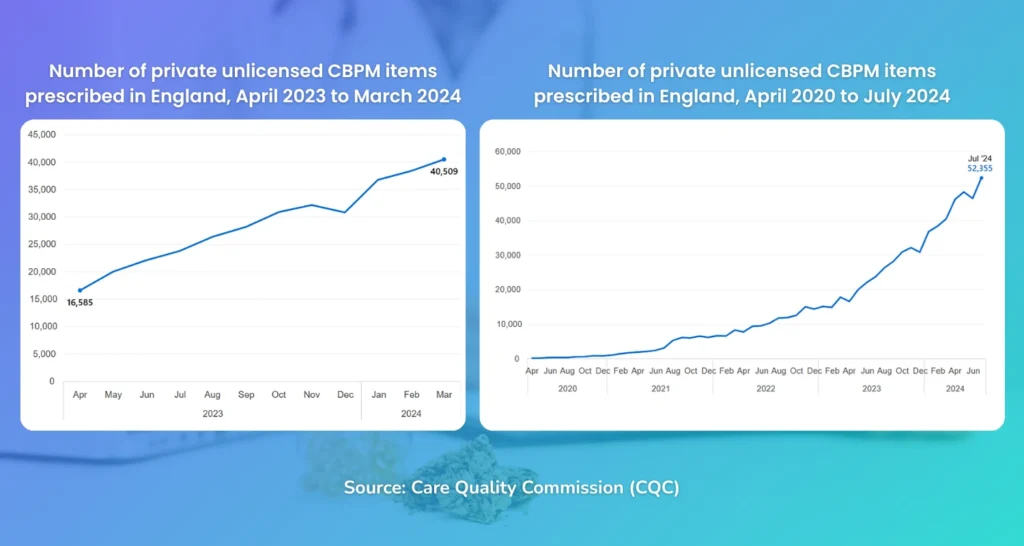
Due to the difficulty in obtaining CBPMs from the NHS, many patients have instead opted for sourcing via the private sector. According to a recently released report of 2024 by the Care Quality Commission (CQC):
- 35 independent clinics were prescribing unlicensed CBPMs.
- Almost all prescriptions for CBPMs remain within the private sector.
- Comparing 2023 to 2024, the number of prescribed items jumped by 130% – from 150,527 (April ’22 to March ’23) to 346,600 (April ’23 to March ’24)
- A clear upward trend in number of prescriptions, especially in the last 3 years.
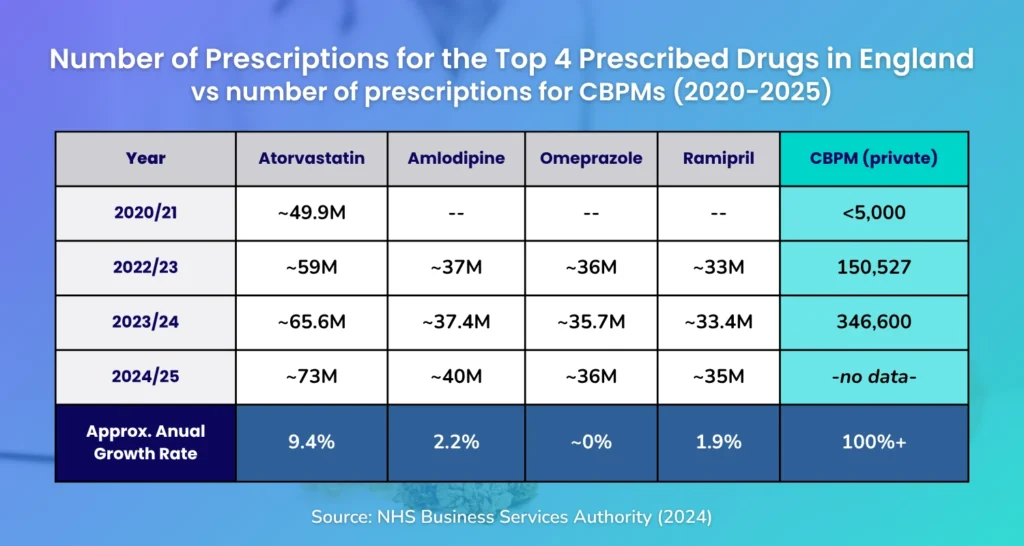
CBPMs exceed percentage growth of mainstream drugs
As seen in the table below comparing the annual number of prescription of CBPMs against the most prescribed drugs in the UK, from 2020 to 2024:
CBPM prescriptions, driven by the private sector, are indeed growing at a faster percentage rate (doubling annually or around 80 % CAGR across 2021–2023), dwarfing percentage growth of mainstream drugs such as Atorvastatin, Amlodipine, Omeprazole, and Ramipril.
But in absolute volume, CBPM prescriptions remain minuscule compared to the millions of prescriptions for statins, PPIs, and antihypertensives.
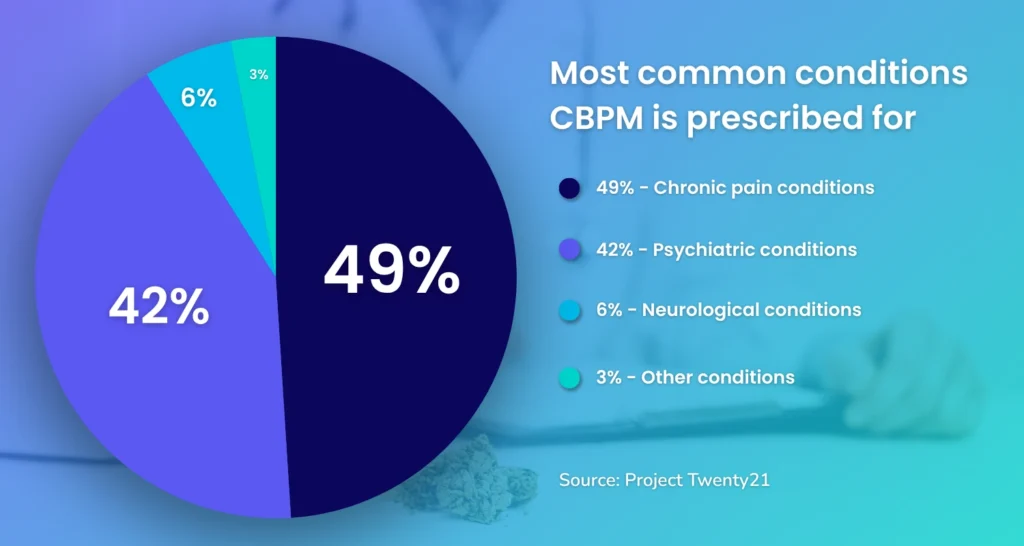
What are CBPMs most commonly prescribed for?
According to a 2023 article by The Pharmaceutical Journal referencing a Project Twenty21 study, the most common conditions CBPMs are prescribed for in the private sector are for chronic pain (e.g., back and/or neck pain, arthritis) and psychiatric conditions (e.g., PTSD, ADHD)
These findings align with Lumino’s data based on the number of placements we’ve made for psychologists and neurologists in the UK over the past 4 years.
Is more current data available?
No. At the moment, these are the most current figures available.
CBPMs are not included in the Dictionary of medicines and devices (dm+d) – as such, prescriptions aren’t compatible with the NHS’s Electronic Prescription Service (EPS) and must be processed manually; hence, the time lag in the availability of prescription data.
The Care Quality Commission's (CQC) concerns regarding CBPMs
Despite the evident growth in CBPM prescriptions, there remain several persistent issues that can lead to the potential harm of patients, according to a CQC report.
1. Lack of Communication & Coordination
Some providers have failed to share treatment plans with other healthcare professionals, including patients’ GPs. This lack of coordination can put patients at risk, especially when CBPMs are involved in complex care plans that require input from multiple specialists.
Secondly, clinics often fail to inform patients on how to prove they are in legal possession of a CBPM. As such, clinics should offer patients clear guidance during the CBPM prescription process. The NHS has published information on this – available in the footnotes of this blog article.
2. Questionable Safety and Legality of Current Prescriptions
Most non-medical prescribing is done by pharmacists, so the first concern here is if all pharmacy prescribers do indeed work within their scope of practice (as highlighted in this guide by the General Pharmaceutical Council).
Secondly, by law, medical specialists should have oversight on patients’ care – including prescriptions, so any services that prescribe CBPMs must certify for themselves that these medical specialists are exercising due diligence in the practice of this oversight.
Thirdly, it is essential that a peer review be conducted before prescriptions are made. This requires having a multi-disciplinary team comprising the relevant specialists to approve prescriptions – particularly for complex cases. Unfortunately, some clinics and small providers still fall short in these areas, and lack the appropriate specialisms in their team to make well-rounded decisions. Furthermore, such incidents are not always being reported as required.
As such, the CQC urges all providers to advise the relevant NHS England CDAO of any incident or concern that occurs, through the Controlled drug reporting tool.
3. Must be an "Unmet Clinical Need"
CBPMs should strictly be prescribed when there’s a verifiable unmet clinical need.
According to the CQC, CBPM prescriptions are made for a wide variety of medical conditions – some of these having arguably little evidence to justify CBPM use.
Some clinics suggest patients need to have tried two prior treatments, but this threshold isn’t always enough. Additionally, clear documentation is critical before prescribing and during the prescription (i.e., recorded in clinical notes).
4. Ethical Advertising
CBPMs are unlicensed and prescription-only, which means they cannot be promoted like over-the-counter products. Furthermore, providers must follow MHRA advertising rules.
Access the CBPM E-Learning Package
In 2018, NHS England, Health Education England, and the University of Birmingham created an e-learning package on medicinal cannabis, covering its pharmacology, legislation, therapeutic uses, and supporting evidence, accessible to all healthcare professionals.
You can access it via this link: https://www.e-lfh.org.uk/programmes/cannabis-based-products-for-medicinal-use/
CBPM criteria under the Regulation 2(1) of the Misuse of Drugs Regulations 2001
Below is an excerpt from Gowling WLG’s summary of the NHS’s guidance & advice on CBPMs.
A cannabis-based product for medicinal use in humans (CBPM) is defined in Regulation 2(1) of the Misuse of Drugs Regulations 2001 (the 2001 Regulations). In summary, a CBPM must meet the following criteria:
It must contain cannabis, cannabis resin, cannabinol, or a cannabinol derivative;
It must be produced for medicinal use in humans; and
It must either be a medicinal product (in regulatory terms) or a component of a medicinal product.
From 1 November 2018, the introduction of Regulation 16A into the 2001 Regulations provided that CBPMs are exempt from certain restrictions that would otherwise apply to controlled drugs under the Misuse of Drugs Act.
Regulation 16A does not represent a general relaxation of controls on CBPMs in England. Rather, it permits specific exemptions to the prohibitions in the Act, allowing the ordering or supply of a CBPM for administration in the following three situations:
The CBPM holds a marketing authorisation (i.e. it is a licensed medicine). For example, NHS England identifies cannabidiol oral solution (Epidyolex®) as a licensed CBPM, having undergone randomised controlled trials for epilepsy syndromes such as Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex;
The CBPM is used for the purposes of a clinical trial (i.e. as an ‘investigational’ medicinal product). NHS England notes that such products are subject to assessment by the Medicines and Healthcare Products Regulatory Agency (MHRA) in relation to safety, quality, and efficacy—unlike unlicensed medicines; or
The CBPM is administered under the prescription or direction of a ‘specialist medical practitioner’.
The third scenario is broader in scope, as it permits the supply of any CBPM on the prescription or direction of a specialist medical practitioner. NHS England notes that “there is no restriction in law for which indications CBPMs may be prescribed,” and that “when prescribing CBPMs, it is a clinical decision to determine the most appropriate treatment for a patient.”
Nonetheless, NHS England makes clear that the decision to prescribe a CBPM should not be taken lightly.
How to prove I have a CBPM prescription?
The information below was taken from the guidance provided by the NHS (original article here):
Keep your medicine in its original packaging, as the dispensing label contains important information about the medicine and the person it’s prescribed for.
The dispensing label is the label the pharmacist puts on the medicine packet when they give you your prescription medicine.
You should also keep a copy of your prescription, and a letter (if you have one) from the doctor who prescribed the medicine. The letter should include your personal details (including name and address), as well as the prescribing doctor’s name and contact details.
You may need to show ID that matches the details on the dispensing label and the prescription, or the doctor’s letter, if you have one.
Acceptable forms of ID include:
- valid passport
- photo driving licence
- proof of age card, such as a PASS card from the national Proof of Age Standards Scheme





